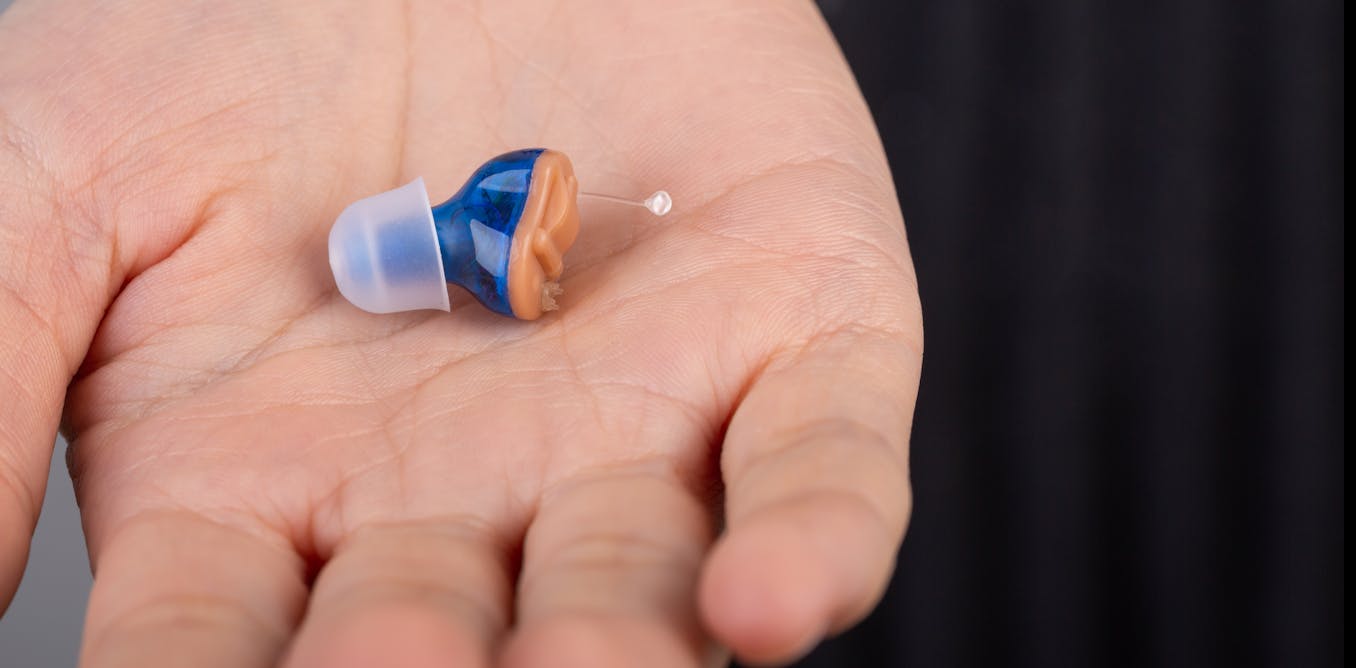
Over-the-counter hearing aids have been greenlighted by the FDA – your local pharmacist will soon be able to sell you the device you need
OTC hearing aids promise to increase the accessibility and affordability of the devices for millions of adults who live with untreated mild to moderate hearing loss.
Aug. 23, 2022 • ~8 min