Acriflavinium_chloride
Acriflavine
Chemical compound
Acriflavine (INN: acriflavinium chloride) is a topical antiseptic. It has the form of an orange or brown powder. It may be harmful in the eyes or if inhaled. It is a dye and it stains the skin and may irritate. The hydrochloride form is more irritating than the neutral form. It is derived from acridine. Commercial preparations are often mixtures with proflavine.[1] It is known by a variety of commercial names.
This article needs more reliable medical references for verification or relies too heavily on primary sources. (December 2016) |  |
 | |
 Sample of pure acriflavine | |
| Names | |
|---|---|
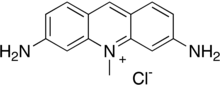
| Preferred IUPAC name
3,6-Diamino-10-methylacridin-10-ium chloride | |
| Other names
Acriflavinium chloride (INN) | |
| Identifiers | |
| |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.211.047 |
| EC Number |
|
PubChem CID |
|
| UNII |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C14H14ClN3 | |
| Molar mass | 259.74 g·mol−1 |
| Pharmacology | |
| R02AA13 (WHO) QG01AC90 (WHO) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Medical use
Acriflavine was developed in 1912 by Paul Ehrlich, a German medical researcher, and was used during the First World War against sleeping sickness and as a topical antiseptic.[2]
Other uses
Acriflavine is used in biochemistry for fluorescently labeling high molecular weight RNA.[1]
It is used as treatment for external fungal infections of aquarium fish.[3]
In an animal model, acriflavine has been shown to inhibit HIF-1, which prevents blood vessels growing to supply tumors with blood and interferes with glucose uptake and use.[4]
Acriflavine might be effective in fighting common cold virus, and also aid the fight against increasingly antibiotic resistant bacteria [5][6][7] because it can cure (remove) plasmids containing antimicrobial resistance genes from Gram positive bacteria.[8]
Since 2014, acriflavine has been undergoing testing as an antimalarial drug to treat parasites with resistance to quinine and modern anti-parasitic medicines.[9]
Australia
Acriflavine is a controlled substance in Australia and dependent on situation,[clarification needed] is considered either a Schedule 5 (Caution) or Schedule 7 (Dangerous Poison) substance. The use, storage and preparation of the chemical is subject to strict state and territory laws.[citation needed]
- "Acriflavine use in aquaria". Archived from the original on 2016-01-29. Retrieved 2016-01-24.
- Lee, K.; Zhang, H.; Qian, D. Z.; Rey, S.; Liu, J. O.; Semenza, G. L. (2009). "Acriflavine inhibits HIF-1 dimerization, tumor growth, and vascularization". Proceedings of the National Academy of Sciences. 106 (42): 17910–5. Bibcode:2009PNAS..10617910L. doi:10.1073/pnas.0909353106. PMC 2764905. PMID 19805192.
- "This forgotten WWI antiseptic could be the key to fighting antibiotic resistance". Science Alert. November 30, 2016.
- Pépin, Geneviève; Nejad, Charlotte; Thomas, Belinda J; Ferrand, Jonathan; McArthur, Kate; Bardin, Philip G; Williams, Bryan RG; Gantier, Michael P (2016). "Activation of cGAS-dependent antiviral responses by DNA intercalating agents". Nucleic Acids Research. 45 (1): 198–205. doi:10.1093/nar/gkw878. PMC 5224509. PMID 27694309.
- Mesas, J.M.; Rodriguez, M.C.; Alegre, M.T. (2004). "Plasmid curing of Oenococcus oeni". Plasmid. 51 (1): 37–40. doi:10.1016/S0147-619X(03)00074-X. PMID 14711527.
- Dana, Srikanta; Prusty, Dhaneswar; Dhayal, Devender; Gupta, Mohit Kumar; Dar, Ashraf; Sen, Sobhan; Mukhopadhyay, Pritam; Adak, Tridibesh; Dhar, Suman Kumar (2014). "Potent Antimalarial Activity of Acriflavine In Vitroand In Vivo". ACS Chemical Biology. 9 (10): 2366–73. doi:10.1021/cb500476q. PMC 4201339. PMID 25089658.
 Media related to Acriflavine at Wikimedia Commons
Media related to Acriflavine at Wikimedia Commons- ChemExper Chemical Directory