Carbon–hydrogen_bond
Carbon–hydrogen bond
Covalent chemical bond between hydrogen and carbon atoms
In chemistry, the carbon-hydrogen bond (C−H bond) is a chemical bond between carbon and hydrogen atoms that can be found in many organic compounds.[1] This bond is a covalent, single bond, meaning that carbon shares its outer valence electrons with up to four hydrogens. This completes both of their outer shells, making them stable.[2]
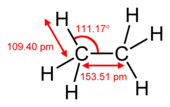
Carbon–hydrogen bonds have a bond length of about 1.09 Å (1.09 × 10−10 m) and a bond energy of about 413 kJ/mol (see table below). Using Pauling's scale—C (2.55) and H (2.2)—the electronegativity difference between these two atoms is 0.35. Because of this small difference in electronegativities, the C−H bond is generally regarded as being non-polar. In structural formulas of molecules, the hydrogen atoms are often omitted. Compound classes consisting solely of C−H bonds and C−C bonds are alkanes, alkenes, alkynes, and aromatic hydrocarbons. Collectively they are known as hydrocarbons.
In October 2016, astronomers reported that the very basic chemical ingredients of life—the carbon-hydrogen molecule (CH, or methylidyne radical), the carbon-hydrogen positive ion (CH+) and the carbon ion (C+)—are the result, in large part, of ultraviolet light from stars, rather than in other ways, such as the result of turbulent events related to supernovae and young stars, as thought earlier.[3]