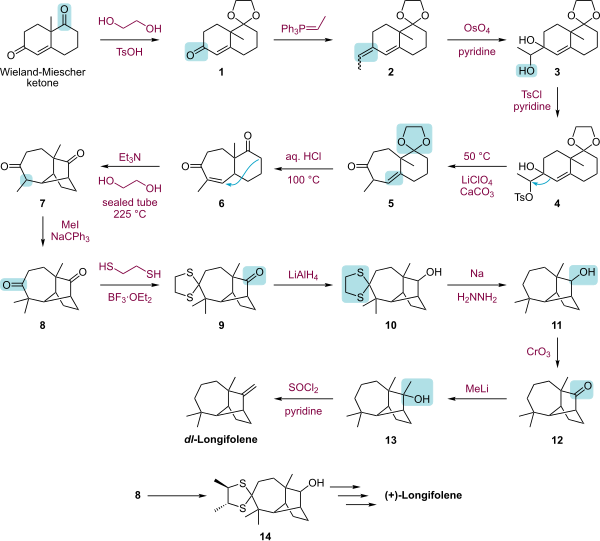
Longifolene
Longifolene
Chemical compound
Longifolene is the common (or trivial) chemical name of a naturally occurring, oily liquid hydrocarbon found primarily in the high-boiling fraction of certain pine resins. The name is derived from that of a pine species from which the compound was isolated,[1] Chemically, longifolene is a tricyclic sesquiterpene. This molecule is chiral, and the enantiomer commonly found in pines and other higher plants exhibits a positive optical rotation of +42.73°. The other enantiomer (optical rotation −42.73°) is found in small amounts in certain fungi and liverworts.
Longifolene is also one of two most abundant aroma constituents of lapsang souchong tea, because the tea is smoked over pinewood fires.[2]