Silver_azide
Silver azide
Chemical compound
Silver azide is the chemical compound with the formula AgN3. It is a silver(I) salt of hydrazoic acid. It forms a colorless crystals. Like most azides, it is a primary explosive.
| Names | |
|---|---|
| IUPAC name
Silver(I) azide | |
| Other names
Argentous azide | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider |
|
| ECHA InfoCard | 100.034.173 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| AgN3 | |
| Molar mass | 149.888 g/mol |
| Appearance | colorless crystals |
| Density | 4.42 g/cm3 |
| Melting point | 250 °C (482 °F; 523 K) explosive |
| Boiling point | decomposes |
| Solubility in other solvents | 2.0×10−8 g/L |
| Structure | |
| Orthorhombic oI16[1] | |
| Ibam, No 72 | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards |
Very toxic, explosive |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Silver azide can be prepared by treating an aqueous solution of silver nitrate with sodium azide.[2] The silver azide precipitates as a white solid, leaving sodium nitrate in solution.
X-ray crystallography shows that AgN3 is a coordination polymer with square planar Ag+ coordinated by four azide ligands. Correspondingly, each end of each azide ligand is connected to a pair of Ag+ centers. The structure consists of two-dimensional AgN3 layers stacked one on top of the other, with weaker Ag–N bonds between layers. The coordination of Ag+ can alternatively be described as highly distorted 4 + 2 octahedral, the two more distant nitrogen atoms being part of the layers above and below.[3]
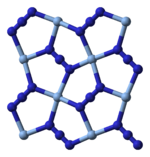 |  | 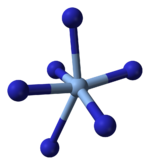 |  |
| Part of a layer | Layer stacking | 4 + 2 coordination of Ag+ | 2 + 1 coordination of N in N−3 |
In its most characteristic reaction, the solid decomposes explosively, releasing nitrogen gas:
- 2 AgN3(s) → 3 N2(g) + 2 Ag(s)
The first step in this decomposition is the production of free electrons and azide radicals; thus the reaction rate is increased by the addition of semiconducting oxides.[4] Pure silver azide explodes at 340 °C, but the presence of impurities lowers this down to 270 °C.[5] This reaction has a lower activation energy and initial delay than the corresponding decomposition of lead azide.[6]
AgN3, like most heavy metal azides, is a dangerous primary explosive. Decomposition can be triggered by exposure to ultraviolet light or by impact.[2] Ceric ammonium nitrate [NH4]2[Ce(NO3)6] is used as an oxidising agent to destroy AgN3 in spills.[5]
- Marr H.E. III.; Stanford R.H. Jr. (1962). "The unit-cell dimensions of silver azide". Acta Crystallographica. 15 (12): 1313–1314. Bibcode:1962AcCry..15.1313M. doi:10.1107/S0365110X62003497.
- Robert Matyas, Jiri Pachman (2013). Primary Explosives (1st ed.). Springer. p. 93. ISBN 978-3-642-28435-9.
- Schmidt, C. L. Dinnebier, R.; Wedig, U.; Jansen, M. (2007). "Crystal Structure and Chemical Bonding of the High-Temperature Phase of AgN3". Inorganic Chemistry. 46 (3): 907–916. doi:10.1021/ic061963n. PMID 17257034.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Andrew Knox Galwey; Michael E. Brown (1999). Thermal decomposition of ionic solids (vol.86 of Studies in physical and theoretical chemistry. Elsevier. p. 335. ISBN 978-0-444-82437-0.
- Margaret-Ann Armour (2003). Hazardous laboratory chemicals disposal guide, Environmental Chemistry and Toxicology (3rd ed.). CRC Press. p. 452. ISBN 978-1-56670-567-7.
- Jehuda Yinon; Shmuel Zitrin (1996). Modern Methods and Applications in Analysis of Explosives. John Wiley and Sons. pp. 15–16. ISBN 978-0-471-96562-6.