Mequitazine
Mequitazine
Chemical compound
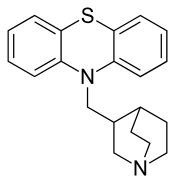
Mequitazine (trade name Primalan) is an H1 antagonist and anticholinergic of the phenothiazine chemical class. It is used to treat allergies and rhinitis.
This article may require cleanup to meet Wikipedia's quality standards. The specific problem is: Convert long prose list(s) to bulleted list(s). (March 2021) |
 | |
| Clinical data | |
|---|---|
| Trade names | Primalan |
| AHFS/Drugs.com | International Drug Names |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank |
|
| ChemSpider |
|
| UNII | |
| KEGG |
|
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.045.005 |
| Chemical and physical data | |
| Formula | C20H22N2S |
| Molar mass | 322.47 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
It was patented in 1969 and came into medical use in 1976.[2]
Severe liver disease; premature infants or full-term neonates.
Pregnancy, lactation; severe cardiovascular disorders; asthma; angle-closure glaucoma, urinary retention, prostatic hyperplasia, pyloroduodenal obstruction; renal and hepatic impairment; elderly, children; epilepsy. May impair ability to drive or operate machinery.
CNS depression including slight drowsiness to deep sleep, lassitude, dizziness, incoordination. Headache, psychomotor impairment and antimuscarinic effects. Rarely, rashes and hypersensitivity reactions, blood disorders, convulsions, sweating, myalgia, paraesthesias, extrapyramidal effects, tremor, confusion, sleep and GI disturbances, tinnitus, hypotension, hair loss. Photosensitivity, jaundice.
Enhances effects of CNS depressants e.g. alcohol, barbiturates, hypnotics, opioid analgesics, anxiolytics and antipsychotics. Can mask signs of ototoxicity caused by aminoglycosides. QT prolongation (which can lead to torsades de pointes arrhythmia) reported with spiramycin.
Same precursor as for Quifenadine. Note that the synthesis has changed over the years from the original. One route seems to involve a Johnson–Corey–Chaykovsky reaction of the starting ketone, although another secondary route is also discussed.
- "Active substance: mequitazine" (PDF). List of nationally authorised medicinal products. Amsterdam: European Medicines Agency. 15 October 2020.
- Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 548. ISBN 9783527607495.
- US 4546185, Bondiou JC, Hodac F, Legroux D, issued Pharmuka Laboratoires
- Gonnot V, Nicolas M, Mioskowski C, Baati R (November 2009). "Expedient synthesis of mequitazine an antihistaminic drug by palladium catalyzed allylic alkylation of sodium phenothiazinate". Chemical & Pharmaceutical Bulletin. 57 (11): 1300–2. doi:10.1248/cpb.57.1300. PMID 19881287.
- Guminski Y, Fabre V, Lesimple P, Imbert T (June 1999). "An efficient synthesis of mequitazine". Organic Preparations and Procedures International. 31 (3): 319–323. doi:10.1080/00304949909458326.
- Leroux S, Larquetoux L, Nicolas M, Doris E (July 2011). "Asymmetric synthesis of (+)-mequitazine from quinine". Organic Letters. 13 (13): 3549–51. doi:10.1021/ol2012567. PMID 21657243.
- Ramírez Chanona N, del Rio Navarro BE, Pérez Martín J (November–December 2005). "[Efficacy of mequitazine (Primalan) on the relief of symptoms of allergic rhinoconjunctivitis in children. Documented clinical experience]". Revista Alergia Mexico (in Spanish). 52 (6): 221–225. PMID 16568706.
- Theunissen EL, Vermeeren A, van Oers AC, van Maris I, Ramaekers JG (February 2004). "A dose-ranging study of the effects of mequitazine on actual driving, memory and psychomotor performance as compared to dexchlorpheniramine, cetirizine and placebo". Clinical and Experimental Allergy. 34 (2): 250–258. doi:10.1111/j.1365-2222.2004.01874.x. PMID 14987305. S2CID 23019669.
- Nakamura K, Yokoi T, Kodama T, Inoue K, Nagashima K, Shimada N, et al. (February 1998). "Oxidation of histamine H1 antagonist mequitazine is catalyzed by cytochrome P450 2D6 in human liver microsomes" (PDF). The Journal of Pharmacology and Experimental Therapeutics. 284 (2): 437–442. PMID 9454781.
- Persi L, Dupin O, Arnaud B, Trinquand C, Michel FB, Bousquet J (April 1997). "Efficacy of mequitazine in comparison with placebo assessed by ocular challenge with allergen in allergic conjunctivitis". Allergy. 52 (4): 451–454. doi:10.1111/j.1398-9995.1997.tb01028.x. PMID 9188930. S2CID 34785561.