MIT team creates ultracold molecules | MIT News
At near absolute zero, molecules may start to exhibit exotic states of matter.
The air around us is a chaotic superhighway of molecules whizzing through space and constantly colliding with each other at speeds of hundreds of miles per hour. Such erratic molecular behavior is normal at ambient temperatures.
But scientists have long suspected that if temperatures were to plunge to near absolute zero, molecules would come to a screeching halt, ceasing their individual chaotic motion and behaving as one collective body. This more orderly molecular behavior would begin to form very strange, exotic states of matter — states that have never been observed in the physical world.
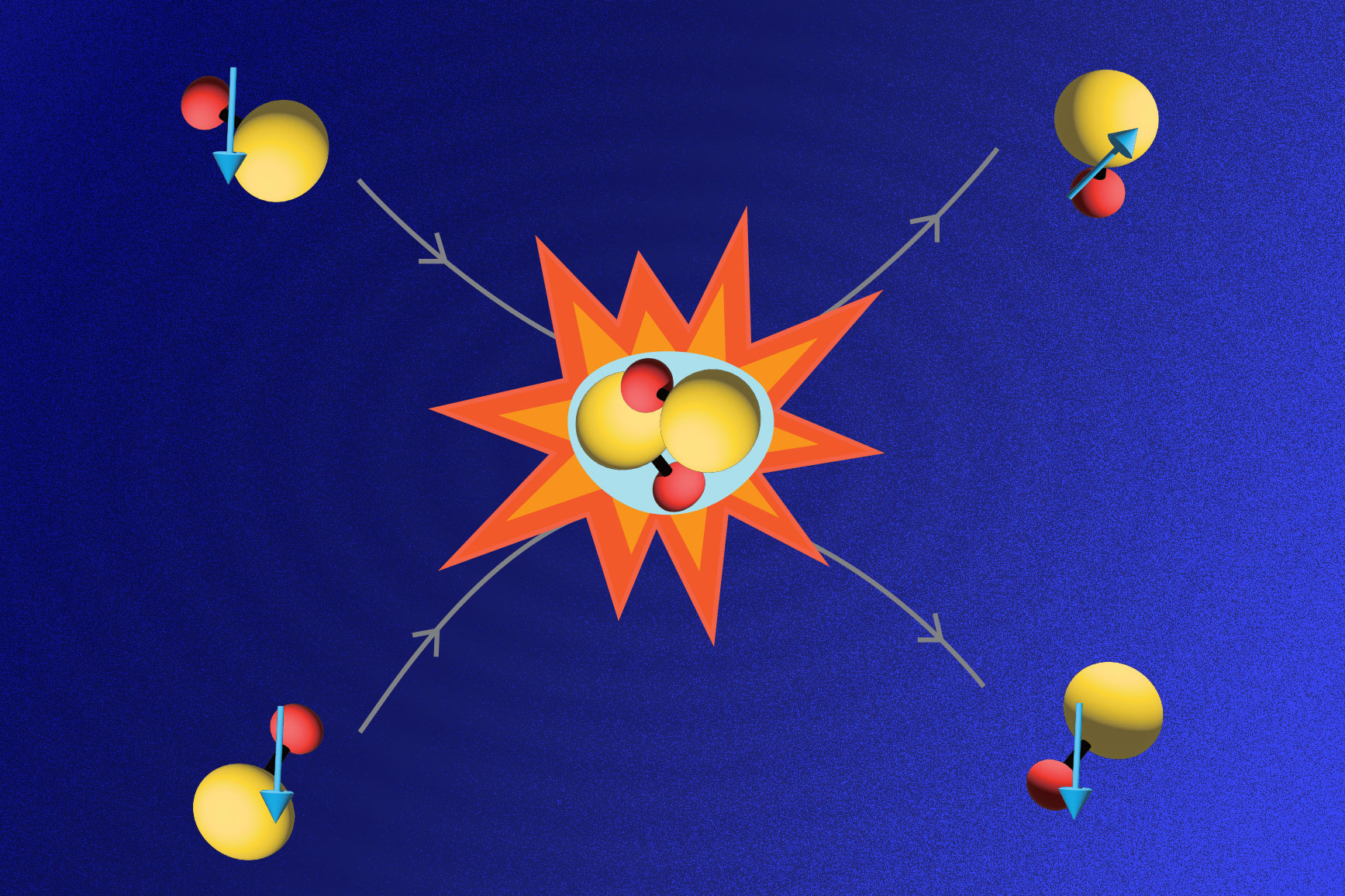
Now experimental physicists at MIT have successfully cooled molecules in a gas of sodium potassium (NaK) to a temperature of 500 nanokelvins — just a hair above absolute zero, and over a million times colder than interstellar space. The researchers found that the ultracold molecules were relatively long-lived and stable, resisting reactive collisions with other molecules. The molecules also exhibited very strong dipole moments — strong imbalances in electric charge within molecules that mediate magnet-like forces between molecules over large distances.
Martin Zwierlein, professor of physics at MIT and a principal investigator in MIT's Research Laboratory of Electronics, says that while molecules are normally full of energy, vibrating and rotating and moving through space at a frenetic pace, the group’s ultracold molecules have been effectively stilled — cooled to average speeds of centimeters per second and prepared in their absolute lowest vibrational and rotational states.
“We are very close to the temperature at which quantum mechanics plays a big role in the motion of molecules,” Zwierlein says. “So these molecules would no longer run around like billiard balls, but move as quantum mechanical matter waves. And with ultracold molecules, you can get a huge variety of different states of matter, like superfluid crystals, which are crystalline, yet feel no friction, which is totally bizarre. This has not been observed so far, but predicted. We might not be far from seeing these effects, so we’re all excited.”
Zwierlein, along with graduate student Jee Woo Park and postdoc Sebastian Will — all of whom are members of the MIT-Harvard Center of Ultracold Atoms — have published their results in the journal Physical Review Letters.
Sucking away 7,500 kelvins
Every molecule is composed of individual atoms that are bonded together to form a molecular structure. The simplest molecule, resembling a dumbbell, is made up of two atoms connected by electromagnetic forces. Zwierlein’s group sought to create ultracold molecules of sodium potassium, each consisting of a single sodium and potassium atom.
However, due to their many degrees of freedom — translation, vibration, and rotation — cooling molecules directly is very difficult. Atoms, with their much simpler structure, are much easier to chill. As a first step, the MIT team used lasers and evaporative cooling to cool clouds of individual sodium and potassium atoms to near absolute zero. They then essentially glued the atoms together to form ultracold molecules, applying a magnetic field to prompt the atoms to bond — a mechanism known as a “Feshbach resonance,” named after the late MIT physicist Herman Feshbach.
“It’s like tuning your radio to be in resonance with some station,” Zwierlein says. “These atoms start to vibrate happily together, and form a bound molecule.”
The resulting bond is relatively weak, creating what Zwierlein calls a “fluffy” molecule that still vibrates quite a bit, as each atom is bonded over a long, tenuous connection. To bring the atoms closer together to create a stronger, more stable molecule, the team employed a technique first reported in 2008 by groups from the University of Colorado, for potassium rubidium (KRb) molecules, and the University of Innsbruck, for non-polar cesium (Ce2) molecules.
For this technique, the newly created NaK molecules were exposed to a pair of lasers, the large frequency difference of which exactly matched the energy difference between the molecule’s initial, highly vibrating state, and its lowest possible vibrational state. Through absorption of the low-energy laser, and emission into the high-energy laser beam, the molecules lost all their available vibrational energy.
With this method, the MIT group was able to bring the molecules down to their lowest vibrational and rotational states — a huge drop in energy.
“In terms of temperature, we sucked away 7,500 kelvins, just like that,” Zwierlein says.
Chemically stable
In their earlier work, the Colorado group observed a significant drawback of their ultracold potassium rubidium molecules: They were chemically reactive, and essentially came apart when they collided with other molecules. That group subsequently confined the molecules in crystals of light to inhibit such chemical reactions.
Zwierlein’s group chose to create ultracold molecules of sodium potassium, as this molecule is chemically stable and naturally resilient against reactive molecular collisions.
“When two potassium rubidium molecules collide, it is more energetically favorable for the two potassium atoms and the two rubidium atoms to pair up,” Zwierlein says. “It turns out with our molecule, sodium potassium, this reaction is not favored energetically. It just doesn’t happen.”
In their experiments, Park, Will, and Zwierlein observed that their molecular gas was indeed stable, with a relatively long lifetime, lasting about 2.5 seconds.
“In the case where molecules are chemically reactive, one simply doesn’t have time to study them in bulk samples: They decay away before they can be cooled further to observe interesting states,” Zwierlein says. “In our case, we hope our lifetime is long enough to see these novel states of matter.”
By first cooling atoms to ultralow temperatures and only then forming molecules, the group succeeded in creating an ultracold gas of molecules, measuring one thousand times colder than what can be achieved by direct cooling techniques.
To begin to see exotic states of matter, Zwierlein says molecules will have to be cooled still a bit further, to all but freeze them in place. “Now we’re at 500 nanokelvins, which is already fantastic, we love it. A factor of 10 colder or so, and the music starts playing.”
This research was supported in part by the National Science Foundation, the Air Force Office of Scientific Research, the Army Research Office, and the David and Lucile Packard Foundation.
What does "over a million times colder than interstellar space" mean? Does "over" mean a 101%, ten times, 10^6? Does "colder than" imply one millionth the temperature? Does interstellar space refer to the cold neutral medium, warm ionized medium, or something else? That would imply that interstellar space is 0.5 Kelvin (times whatever "over" means), which is off by a factor of anywhere from 20 to 20000 to 10^7, depending on your definition of interstellar space. It seems like an uninformative and even misleading phrase to put in this article. [Ferriere, K. (2001), "The Interstellar Environment of our Galaxy", Reviews of Modern Physics 73 (4): 1031–1066]
I would like to note a prior theoretical basis of such novel magnetic bonding, structure, stability and reaction dynamics. This binding of NaK at ultracold temperatures has been modeled based on fermionic relativistic spinrevorbital as involving the fermionic nuclei of the Na and K and the electron pair of their s orbital overlaps. Such was published in " A Theory of the Relativistic Fermionic Spinrevorbital" Seehttp://www.academicjournals.... Obtain free manuscript by click on "Full Text PDF" . On page 21 I note " the lability of s block ions to water exchange s consistent with the Little (Effect) Rules, just as protons and protonlysis are consistent at higher temperatures. The s spinrevorbital (for p block and s block metals) allows the strongest interactions of ligand onor electrons to the nucleus of the metal centers for nuclear spin induced revorbital effects that facilitate ligands entering and leaving dynamics for lability by Rule 2 (of the metal cneter) or protons (during protolsis in acidic media perturb the motions of electron pair by Rule 2 during the coordinate covalent bond rearrangement between the metal center and the ligands during the exchange reactions. The odd nuclear spins of the metal centers can induce disontinuum to continuum activation of lone electrons on the ligands with consequent facile bonding dynamcs and rearrangement of ligands by Rules 2 and 3..." Such bonding dynamics at lower temperatures can magnetically bind the Na and K by their nuclear spin as mediated by the e pair in s orital. The electric dipole of K-Na drives the electrons back and forth to strongly couple the nuclear moments for stability relative to K-K and Rb-Rb and Na-Na. the Na-K exhibit stronger bond at the lower temperature due to the lower mass and the gravity of the bonding relative to K-Rb. I think at slightly higher temperatures (relative to the 50 nanoKrelvins of this experiment) the K-Rb molecule would be more stable than the NaK molecule as the heavier KRb would as by this theory of RBL transform more of the thermal energy at the higher temperature to magneto gravimetric binding of the fermionic nuclei as the higher temperature cause the heavier Rb to move to create stronger magnetic nuclear moment of the Rb so it magnetically binds the K just as the less massive Na moves faster at the lower temperature of 50 nanoKelvin to magnetically bind the moving K fermionic nucleus. The spinrevorbital nature of binding in motions magnetogravimetrically of my theory explains the observed long range nature of the binding in NaK. Note at the higher temperature where the RbK may bind the NaK moves too fast and may be unstable! On page 21 and throughout, this theory of relativistic fermionic spinrevorbital gives a solid basis for explaining this beautiful experiment!
Ok, if they can do this then how about a more efficient refrigerator / freezer! Can lasers be used to create the equivalent of a microwave freezer? Can microwaves be used to create a microwave freezer?
What is a "crystal of light"? "That group subsequently confined the molecules in crystals of light to inhibit such chemical reactions."
Although not a complete answer, one place to start is with the coldest naturally occurring place in the universe, which is the Boomerang Nebula, a planetary nebula that is around 1 K. As best as I can tell, this cooled below the CMB temperature simply by adiabatic expansion, and is insulated in its interior from CMB heating. Is this a feasible way to get to ultracold temperatures?
For a monoatomic gas, recall that adiabatic expansion is TV2/3=const. So something that cools to 100 nK from 3 K would have to expand in volume by a factor of ~1011. Which is, obviously, a lot, but space is big and has more than enough room. The Boomerang nebula is about 1 light-year across and is expanding out at about 164 km/s. So we could imagine, for example, a similar object that starts out with a radius of around 10^(-4) LY (which is still 10,000 times larger than the Sun) and expands to the same size at the same rate, which would take around 1000 years. This doesn't seem particularly implausible, although I'm no astronomer.
The harder question to answer is what the heating rate from the CMB would be in the interior of this cloud. It would only have to be very small, of course, to counteract the adiabatic cooling. Looking at one of the papers on the Boomerang nebula, the authors there estimate the cosmic ray heating as 4∗10−28 erg/s, while the cooling rate is around 10−25 in the same units. So since adiabatic cooling goes slower as the gas gets colder (indeed, in this simple model we have T˙(t)=−T/t), we would probably expect that by the time the gas has cooled to about 1/1000th of the CMB temperature, if not sooner, the heating rate would match the cooling rate.
All in all, my very crude best guess then is that adiabatic expansion of this sort could not lead to a temperature below the mK scale.
Reprinted with permission of MIT News