Ideal_Gas_Law.jpg

|
This image was
uploaded
in the
JPEG
format even though it
consists of non-photographic data
. This information could be stored more efficiently or accurately in the
PNG
or
SVG
format.
If possible, please upload a PNG or SVG version of this image without
compression artifacts
, derived from a non-JPEG source (or with existing artifacts
removed
). After doing so, please tag the JPEG version with
{{Superseded|NewImage.ext}}
and remove this tag. This tag should not be applied to photographs or scans. If this image is a diagram or other image suitable for
vectorisation
, please tag this image with
{{Convert to SVG}}
instead of
{{BadJPEG}}
. If not suitable for vectorisation, use
{{Convert to PNG}}
. For more information, see
{{BadJPEG}}
.
|
|
Summary
| Description Ideal Gas Law.jpg |
English:
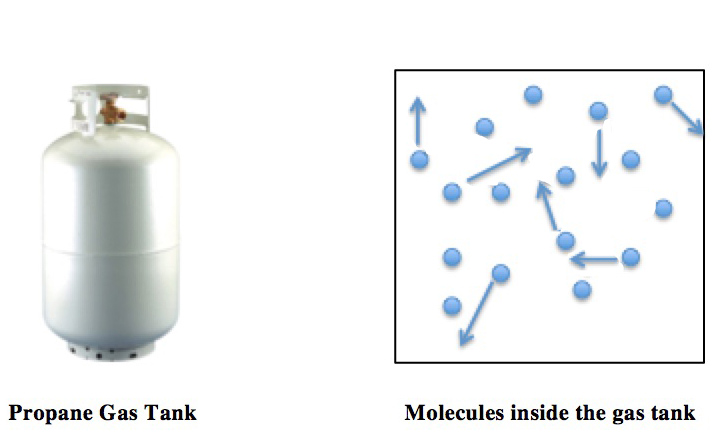
Molecular collisions within a closed container (the propane tank) are shown (right). The arrows represent the random motions and collisions of these molecules. The pressure and temperature of the gas are directly proportional: as the temperature is increased, the pressure of the propane increases by the same factor. A simple consequence of this proportionality is that on a hot summer day, the propane tank pressure will be elevated, and thus propane tanks must be rated to withstand such increases in pressure.
|
| Date | |
| Source | Own work |
| Author | BlyumJ |
Licensing
I, the copyright holder of this work, hereby publish it under the following license:
This file is licensed under the
Creative Commons
Attribution-Share Alike 4.0 International
license.
-
You are free:
- to share – to copy, distribute and transmit the work
- to remix – to adapt the work
-
Under the following conditions:
- attribution – You must give appropriate credit, provide a link to the license, and indicate if changes were made. You may do so in any reasonable manner, but not in any way that suggests the licensor endorses you or your use.
- share alike – If you remix, transform, or build upon the material, you must distribute your contributions under the same or compatible license as the original.
Captions
Add a one-line explanation of what this file represents